Cognitive impairment could be due to Alzheimer's disease, or not.
Knowing can help you plan the path ahead
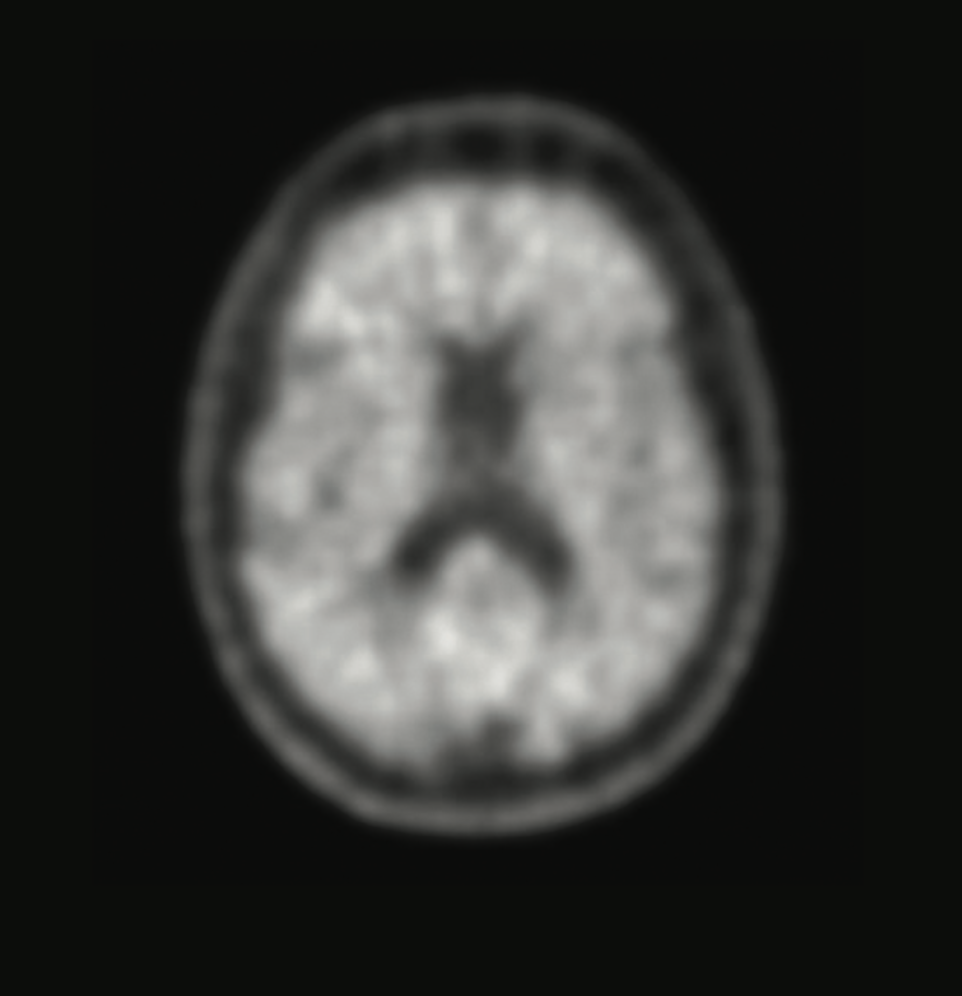
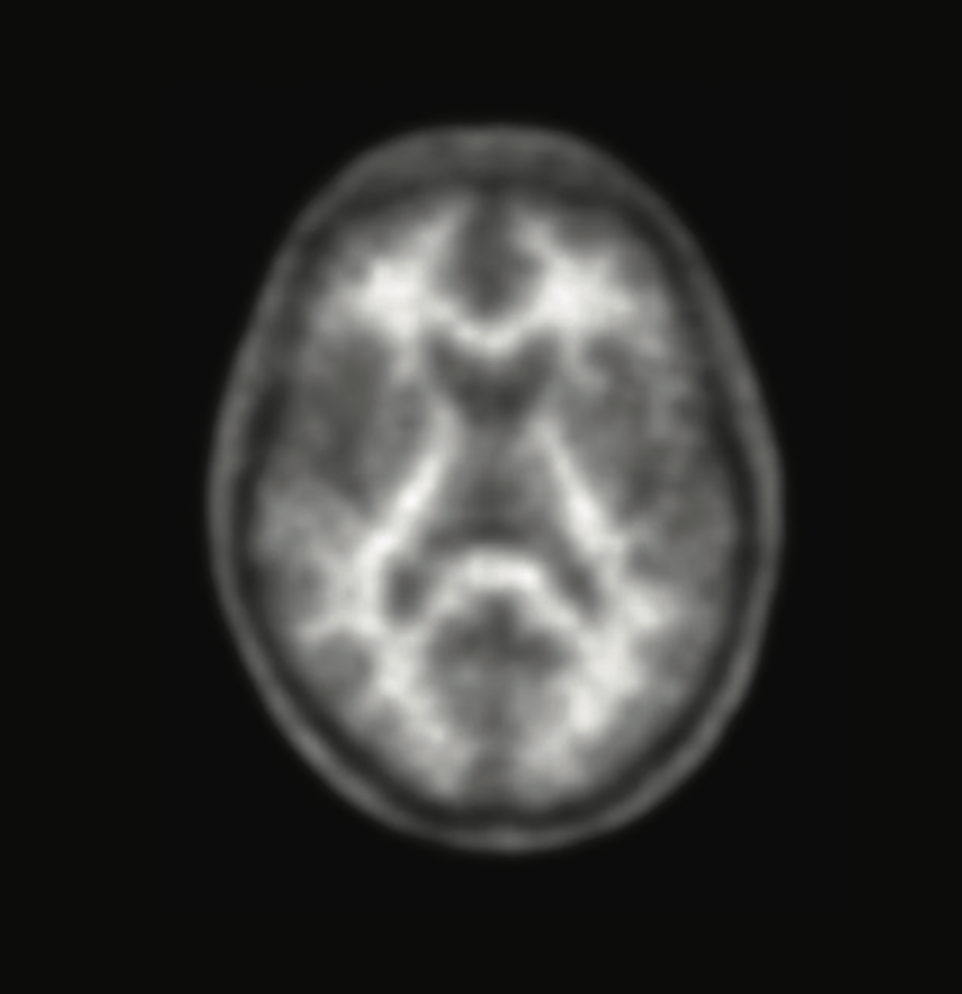
When memory loss begins to affect daily life, knowing the cause is critical. A brain scan with Neuraceq® gives your doctor information that can help assess your condition.