Clarity in Amyloid PET Imaging
An Amyloid PET scan is a key step.
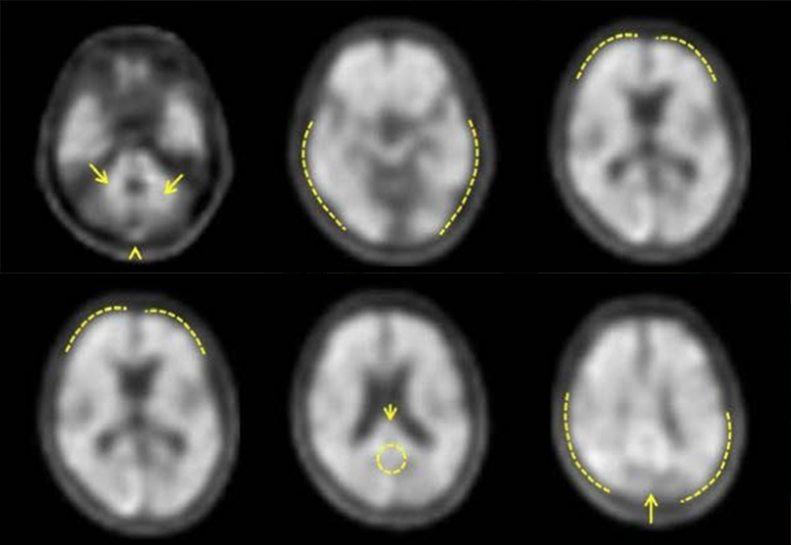
Neuraceq® reliably visualizes β-amyloid neuritic plaques to confirm eligibility for new Alzheimer’s disease therapies
Neuraceq® reliably visualizes β-amyloid neuritic plaques to confirm eligibility for new Alzheimer’s disease therapies


A precise diagnostic tool is needed to reduce the time to accurate diagnosis
The information contained in Neuraceq.com/hcp is intended for healthcare professionals in the United States only. If you are a US healthcare professional, click the "Continue" button below.
Yes, I am a US healthcare professional and would like to continue.
This will close in 0 seconds
PRODUCT INDICATIONS AND USE: Neuraceq is indicated for Positron Emission Tomography (PET) imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer's Disease (AD) and other causes of cognitive decline. A negative Neuraceq scan indicates sparse to no neuritic plaques and is inconsistent with a neuropathological diagnosis of AD at the time of image acquisition; a negative scan result reduces the likelihood that a patient's cognitive impairment is due to AD. A positive Neuraceq scan indicates moderate to frequent amyloid neuritic plaques; neuropathological examination has shown this amount of amyloid neuritic plaque is present in patients with AD, but may also be present in patients with other types of neurologic conditions as well as older people with normal cognition. Neuraceq is an adjunct to other diagnostic evaluations.
Limitations of Use:
A positive Neuraceq scan does not establish the diagnosis of AD or any other cognitive disorder. The safety and effectiveness of Neuraceq have not been established for Predicting the development of dementia or other neurologic conditions or monitoring responses to therapies.
IMPORTANT SAFETY INFORMATION:
CONTRAINDICATIONS: None
WARNINGS AND PRECAUTIONS:
ADVERSE REACTIONS:
DRUG INTERACTIONS:
USE IN SPECIFIC POPULATIONS:
OVERDOSAGE:
A pharmacological overdose of Neuraceq is unlikely given the relatively low doses used for diagnostic purposes. In the event of administration of a radiation overdose with Neuraceq, the absorbed organ dose to the patient should be reduced by increasing elimination of the radionuclide from the body by inducing frequent micturition. Prior to Neuraceq administration, please read the full Prescribing Information for additional Important Safety Information.
SUSPECTED ADVERSE REACTIONS: please report to: https://www.fda.gov/safety/medwatch-fda-safety-information-and-adverse-event-reporting-program
