Neuraceq® delivers sensitive and reliable detection of β-amyloid neuritic plaques
Neuraceq® is an accurate diagnostic tool to aid in the evaluation of cognitive decline.1 Neuraceq® reveals β-amyloid neuritic plaques in different brain regions, thereby allowing an estimate of plaque density by degree of uptake.1
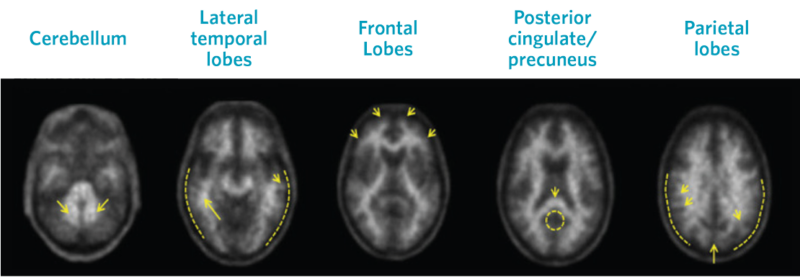
Negative Scans
Top row images are negative scans, corresponding to lower tracer uptake in gray matter than white matter, and reduced likelihood that cognitive decline is a result of AD
× .
negative-1
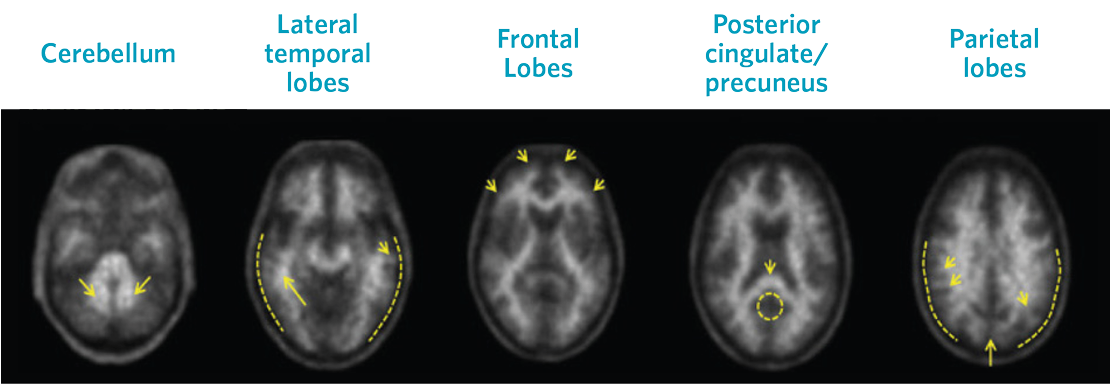
Visual assessment is based on the systematic comparison of tracer uptake in gray and white matter, focusing on specific cortical regions, as seen in greyscale images.
1Neuraceq® (florbetaben F18 injection) prescribing information. Life Molecular Imaging; June 2025. Fareham, UK.
Positive Scans
Images show positive scans with equal or higher tracer uptake in gray matter than white matter and moderate to frequent β-amyloid neuritic plaques
× .
positive-1
Neuraceq® scan reports can help facilitate dialogue between physicians, patients, and caregivers
While the actual diagnosis is made by reading the black and white scans, useful reports provided to you by your patient’s imaging specialist include an accompanying written assessment.
The Neuraceq® scan is convenient for patients
Neuraceq® provides a wide, stable scan window of 45-130 minutes, and a 15-20 minute scan time, making the process quick and convenient for your patients. A Neuraceq® PET scan involves an injection into a vein in the arm and a short scan.
Neuraceq® injection

Wait 45+ minutes
PET Scan
Scan completed
Frequently Asked Questions about Neuraceq®
Is Neuraceq® currently available?
Neuraceq® was FDA approved in March 2014 and is available in many imaging centers across the US.
Contact Your Rep for specific details on Neuraceq® availability in your area.
What role can this test play in the diagnostic process?
Neuraceq® can give you important information in cases where Alzheimer’s disease is suspected. If the scan is positive, it means that beta amyloid plaques are present in the brain. If the scan is negative, it means that beta amyloid plaques are not present, and another diagnosis can be considered. View the USPI for more information.
How does a positive or negative scan assist in my ability to assess patients?
Early detection and intervention can benefit patients. Having a diagnosis gives patients the opportunity to plan ahead. Pharmacological and non-pharmacological interventions can begin sooner. Knowing a diagnosis gives patients the opportunity to receive treatment with FDA-approved amyloid-targeting therapies.
Do you have any materials I can provide to my patient to help explain the Neuraceq® scan?
Our patient and caregiver information can be viewed on our patient and caregiver page. To request digital or printed materials, simply use the map to find your Neuraceq® representative and reach out directly.
Who can I contact for more information?
Contact Us for more information.

