Quantification – FDA Approved with Neuraceq®
Why Quantification Matters in Today's Evolving Diagnostic Landscape
- Quantification with Neuraceq® offers an objective measurement of β-amyloid plaque burden, supporting clinicians in the evaluation of patients with cognitive impairment.1
- As an adjunct to visual interpretation, quantitative data enhances diagnostic confidence — particularly in cases with subtle uptake.2
- Quantification may aid in identifying patients eligible for amyloid-targeting therapies.1
- By providing a reliable, objective assessment of amyloid burden1,3, quantification can support evaluation of disease across various stages of cognitive impairment and dementia.2
Quantification can enhance diagnostic confidence, particularly in intermediate patient cases3
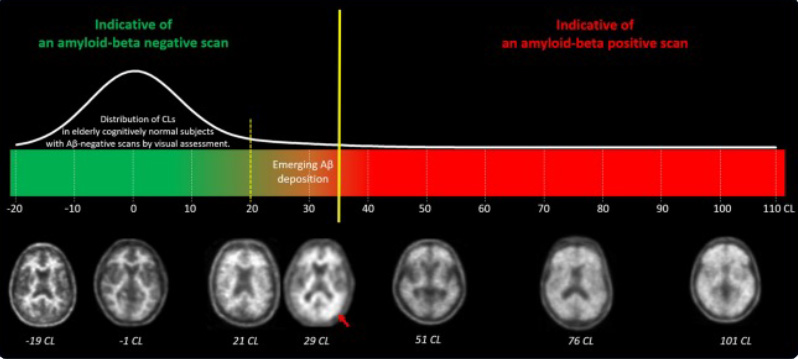
Note: the 20-35 range (the region between the yellow lines) is considered “intermediate.”
Jovalekic, A., Roé-Vellvé, N., Koglin, N., Quintana, M. L., Nelson, A., Diemling, M., Lilja, J., Gomez-Gonzalez, J. P., Dore, V., Bourgeat, P., … & Bullich, S. (2023). Validation of quantitative assessment of florbetaben PET scans as an adjunct to the visual assessment across 15 software methods. European Journal of Nuclear Medicine and Molecular Imaging, 50, 3276–3289. https://doi.org/10.1007/s00259-023-06279-0
-
≥35 CL: Strongly associated with established amyloid pathology and moderate to frequent neuritic plaques.
-
<20 CL: Common in cognitively normal elderly patients, typically corresponding to negative amyloid PET scans.
-
20–35 CL: In these intermediate patient cases, quantitative analysis can provide valuable clarity by highlighting subtle, focal, or unilateral amyloid deposition.
-
Readers should review such scans carefully to identify subtle amyloid accumulation that can be focal and/or unilateral (see the red arrow on the figure for reference)
-
All trademarks, logos and brand names are the property of their respective owners. All company, product and service names used above are for identification purposes only. Use of these names, trademarks and brands does not imply endorsement.
Enhance Your Confidence in Amyloid PET Interpretation
The Neuraceq® Reader Training course now includes an optional quantification module.
*Use the Branch Code FBBReaderTraining if you are not currently registered.
For more information or questions surrounding Quantification with Neuraceq®, contact Your Neuraceq® Representative.
For technical information on quantitative analysis, visit Contact Us and select “Medical Affairs” under Inquiry Type.
References:
- Neuraceq® (florbetaben F18 injection) prescribing information. Life Molecular Imaging; June 2025. Fareham, UK.
- Collij LE et al., J Nucl Medicine 66(1)110-116. (2025). Link: https://pubmed.ncbi.nlm.nih.gov/39542700/
- Jovalekic A et al., Eur J Nucl Med Mol Imaging 50:3276-3289. (2023). Link: https://pubmed.ncbi.nlm.nih.gov/37300571/


